
How to Overcome Depression After Vacation?
How to Overcome Depression After Vacation? Get Instant Relief Now! It’s not uncommon to feel a wave of emotions, including sadness or even tears, after

The recent FDA approval of Esketamine stands as a beacon of hope, promising a transformative breakthrough in the realm of depression management. Esketamine, a derivative of the well-known anesthetic ketamine, has captured attention for its unique approach and marked efficacy, particularly in cases resistant to traditional therapies.
Administered as a convenient nasal spray, esketamine’s approval marks a significant stride toward addressing the persistent challenges associated with depression, offering a novel avenue for those in search of effective and accessible mental health solutions.
Esketamine, a derivative of the well-known anesthetic ketamine, has emerged as a groundbreaking player in the field of mental health treatment. Unlike its predecessor, Esketamine has garnered attention for its profound antidepressant effects, particularly in cases where traditional treatments have fallen short. Administered through a nasal spray, Esketamine offers a user-friendly alternative, making it more accessible to those seeking relief from the burdens of depression.
While the precise mechanisms underlying its antidepressant properties are still being studied, Esketamine’s ability to modulate the neurotransmitter glutamate, particularly through the N-methyl-D-aspartate (NMDA) receptor, has shown promise in restoring neural connectivity and fostering resilience against the debilitating effects of depression.
Esketamine received its game-changing FDA approval on March 5, 2019, marking a pivotal moment in the treatment of depression. This momentous decision came after a series of clinical trials demonstrated the efficacy of Esketamine, particularly in cases of treatment-resistant depression where other options had fallen short.
March 5, 2019: Esketamine gains FDA approval, signaling a new era in mental health treatment.
Clinical Trials: Rigorous studies paved the way for approval, showcasing esketamine’s effectiveness, especially in challenging cases.
Treatment-Resistant Depression: Esketamine’s approval addresses a critical need, offering a lifeline for individuals grappling with depression that doesn’t respond to conventional therapies.
The approval history of ketamine, the parent compound of Esketamine, lays the foundation for understanding the significance of its derivative’s recent FDA approval. Originally approved as an anesthetic in the 1970s, ketamine’s unique properties, including its rapid onset and short duration of action, sparked interest in its potential beyond the operating room.
Fast forward to the present day, and the FDA approval of Esketamine in 2019 represents a significant evolution in the recognition of ketamine’s therapeutic potential, particularly in treating depression. This historical journey illuminates the progressive shift from ketamine’s established use in anesthesia to its groundbreaking role in mental health Esketamine, underscoring the ongoing exploration of novel applications for existing pharmaceuticals.
1970s:
Ketamine was initially approved as an anesthetic, showcasing its safety and efficacy in surgical settings.
Therapeutic Potential:
Ongoing research and clinical trials recognize ketamine’s potential beyond anesthesia, leading to the exploration of mental health treatment.
Esketamine Approval (2019):
The FDA’s approval of Esketamine marks a pivotal moment, acknowledging the antidepressant capabilities derived from ketamine, and opening new doors in the management of depression.
Continued Exploration:
The approval of Esketamine prompts continued exploration of ketamine’s multifaceted applications, fostering a deeper understanding of its potential benefits across various medical domains.
In 2020, the landscape of mental health treatment witnessed a significant continuation of the impact initiated by esketamine’s groundbreaking FDA approval in 2019. This approval, granted on March 5, 2019, paved the way for increased accessibility and utilization of esketamine in addressing treatment-resistant depression.
In the subsequent year, healthcare providers and patients alike experienced the tangible effects of this approval, as Esketamine became an integral component of mental health interventions. The year 2020 marked a period of implementation, adaptation, and further research into Esketamine’s long-term efficacy and potential applications.
Solidifying its status as a promising breakthrough in the ongoing quest for more effective and accessible treatments for mental health conditions.
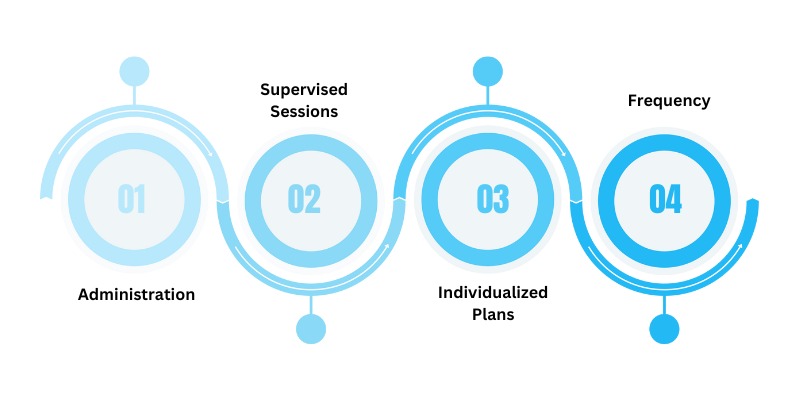
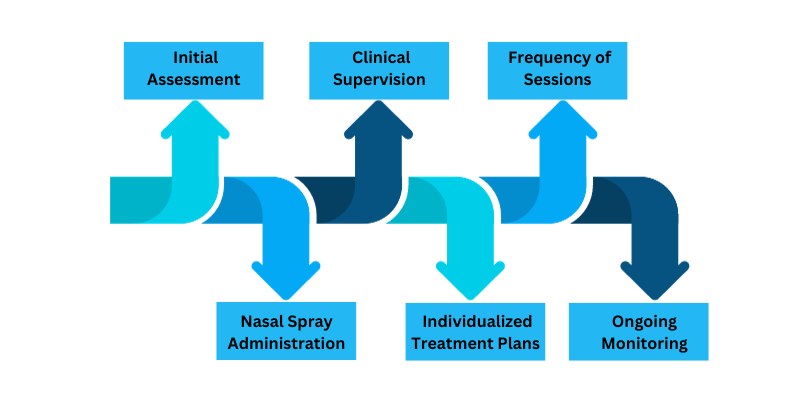
Esketamine treatment introduces a novel and accessible approach to managing depression, offering a distinct experience compared to traditional therapies. Typically administered as a nasal spray, the process is straightforward and minimally invasive. Patients receive the nasal spray under the supervision of a healthcare professional in a clinic setting. The frequency and duration of treatments may vary based on individual needs and treatment plans.
Administration: The nasal spray format enhances ease of use, making it a practical choice for both patients and healthcare providers.
Supervised Sessions: Esketamine treatment sessions are conducted under the watchful eye of a qualified healthcare professional, ensuring safety and proper administration.
Individualized Plans: Treatment plans are tailored to the unique needs of each patient, accounting for the severity of their depression and response to the therapy.
Frequency: The frequency of esketamine treatments may vary, with some patients requiring more frequent sessions initially, followed by a maintenance phase.
Esketamine treatment represents a departure from traditional approaches, offering a promising alternative for those seeking relief from the burdens of depression. The simplicity of its administration and the potential for efficacy make it a notable addition to the spectrum of available mental health interventions.
Esketamine therapy is designed for individuals facing the challenges of treatment-resistant depression or major depressive disorder. It is particularly suitable for those who have not found relief through conventional antidepressant medications.
Candidates for esketamine therapy often include individuals seeking rapid and effective intervention for their depressive symptoms. However, it is crucial for healthcare professionals to assess each case individually to determine the appropriateness of Esketamine therapy based on the severity of the condition, medical history, and response to previous treatments.
Esketamine’s efficacy in treating depression lies in its unique mechanism of action, distinct from traditional antidepressants. As a glutamate receptor modulator, particularly targeting the N-methyl-D-aspartate (NMDA) receptor, Esketamine facilitates the release of certain neurotransmitters, leading to the restoration of synaptic connections and the promotion of neural plasticity.
This process is believed to play a crucial role in alleviating depressive symptoms. The therapy is administered as a nasal spray, allowing for more direct and rapid delivery of the medication into the bloodstream, which contributes to its faster onset of action compared to traditional antidepressants.
Esketamine’s targeted approach and efficient delivery method contribute to its role as a promising and innovative treatment option for depression.
Recent developments in the field of mental health have expanded the scope of esketamine’s application beyond its initial approval for treatment-resistant depression. The new indication for Esketamine involves its use in conjunction with oral antidepressant medications for the treatment of depressive symptoms in adults with major depressive disorder (MDD) who have demonstrated inadequate response to conventional antidepressant therapies alone.
This expansion broadens the accessibility of Esketamine, providing a more comprehensive approach to managing depression by combining the benefits of both traditional oral antidepressants and innovative nasal spray therapy. The new indication reflects a nuanced understanding of the diverse needs of individuals grappling with depression and represents a step forward in tailoring treatment approaches for improved outcomes.
From its roots in the well-established ketamine to the groundbreaking approval in 2019 and subsequent expansions in its indications, Esketamine represents a transformative approach to mental health intervention. With its nasal spray administration, unique mechanism of action, and recent application for major depressive disorder, Esketamine stands at the forefront of innovation, offering a promising avenue for those resistant to conventional treatments.

How to Overcome Depression After Vacation? Get Instant Relief Now! It’s not uncommon to feel a wave of emotions, including sadness or even tears, after

How to Help Someone with Anxiety? Get Instant Relief Now! Anxiety is a common mental health issue that many people experience at some point in

Understanding Shower Avoidance Syndrome: Causes, Symptoms, and Treatment Options Shower Avoidance Syndrome, also known as SAS, is a psychological condition characterized by a persistent aversion
